Four laws of thermodynamics are unavoidable, despite all mystical hopes.
|
0th Law: |
|
1st Law: Energy is conserved. |
|
2nd Law: The entropy of an isolated system never decreases. |
|
3rd Law: The entropy of a pure substance is nil at T= 0 K. |
Sensible heat, heat content or latent heat: this quantity depends on the mass and on the specific heat capacity of the material. Heat involved in a change of its temperature is calculated as:
![]()
Where
- Q is the total heat implied in the change [J]
- m is the mass of the material [kg]
- CP is the specific heat capacity at constant pressure [kJ kg-1 K-1]
Ice at 0 °C 2.05
Water at 15 °C 4.186
Air at -50 to +40 °C 1.005
- T1 and T0 are the temperatures after and before the change [K]
Melting - Crystallisation, Evaporation – Condensation: heat is involved by the change of the physical state of the material.
For climate considerations, water is the vehicle of such processes:
Latent heat of fusion of water at 0 °C: 334 [ kJ kg-1 ]
Latent heat of vaporisation of water at 100 °C and normal pressure: 2257 [ kJ kg-1 ]
Latent heat of vaporisation of water at 14 °C and normal pressure: 2467 [ kJ kg-1 ]
Planck’s Law
Planck’s law defines the radiance, the quantity of radiation that passes through or is emitted from a surface when in thermal equilibrium at a definite temperature.
At a given wavelength the radiance will be calculated as:
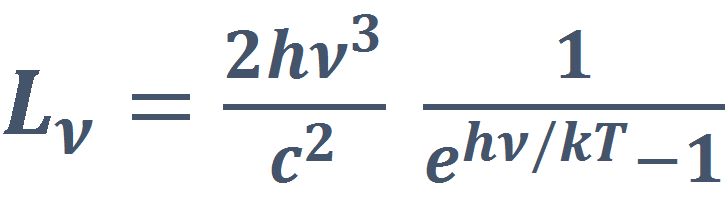 [W m-2 sr-1 Hz-1 = W m-2 sr-1 s]
[W m-2 sr-1 Hz-1 = W m-2 sr-1 s]
where:
- h is Planck’s constant [6.6260693×10−34 W s2]
- c is the speed of light [2.99792458×108 m s−1]
- k is Boltzmann’s constant [1.380658×10−23 J K−1]
- λ/c [s-1], where λ is the wave length [m].
Solving this over a range of wavelengths for a temperature of 5’776 K gives the following figure:
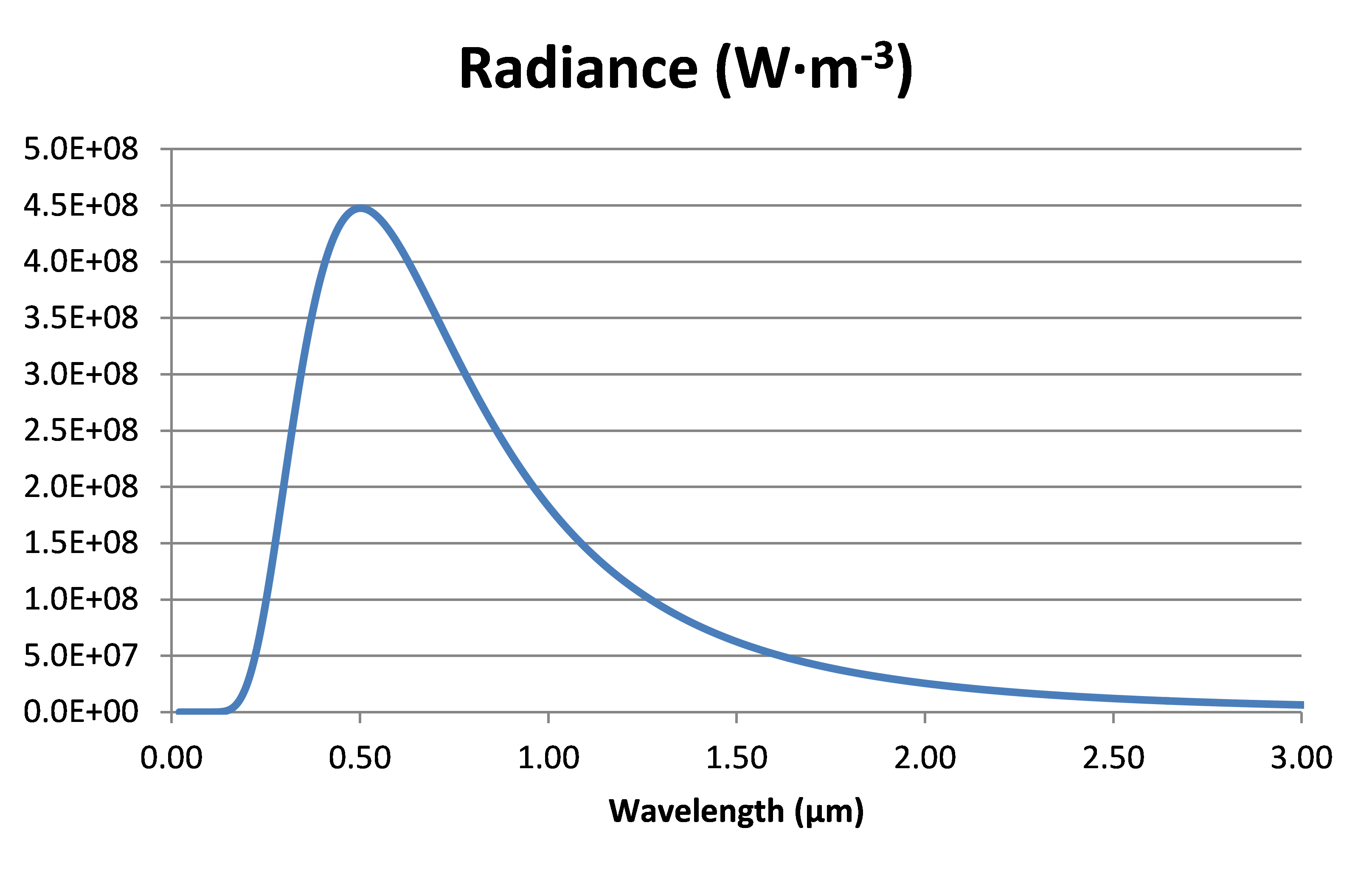
Radiance of a black body at 5776 K, the so-called effective temperature of the sun.
The wavelength at which there is a maximum is calculated by the Wien’s law:
In the case of the sun at 5776 K, λmax = 0.002898/T = 0.502 µm; this is within the visible range of light (0.38-0.75 µm).
At the surface temperature of the Earth of 288 K (15 °C) the radiance spectra will be:
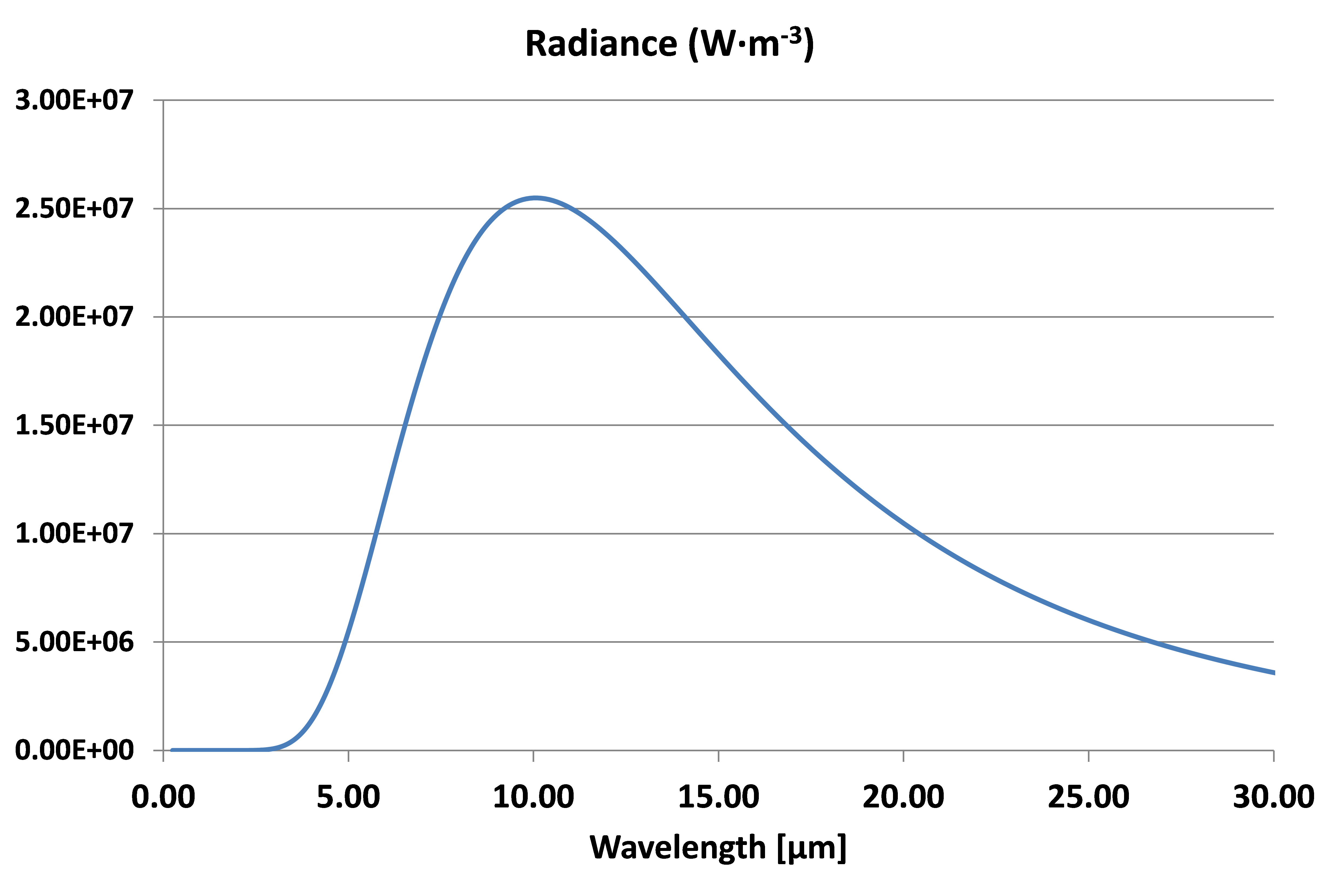
Radiance spectra at T =288 K
In this case the peak is at λmax = 10.06 µm, in the non-visible infrared range.
Stefan Boltzman Law
This laws integrates Plank’s law and defines the electromagnetic power that is emitted by the surface of a lump of matter in relation with its temperature.
![]()
Where
- E is the flux of energy [W m-2]
- ε is the emissivity of the black body (1 if perfect) [-]
- σ is the Stefan Boltzman constant 5.670·10-8 [W· m-2· K-4]
- T is the temperature [K]
Thus at 5’776 K the sun emits 63.11 MW m-2.
And the Earth, at its hypothetical 288 K and if a perfect black body,which it is not, emits 390 W m-2.
Far away from us, at the temperature of the cosmic microwave background radiation of 2.7 K,
the radiation is a mere 3 µW·m-2.
The emissivity factor of 1 applies only for a hypothetical perfect black body. Reality of matter is other:
| Material | Emissivity |
|---|---|
| Aluminium (anodized) | 0.77 |
| Aluminium (polished) | 0.039 - 0.057 |
| Asphalt | 0.93 |
| Carbon (graphite) | 0.98 |
| Clay (baked) | 0.91 |
| Concrete | 0.92 |
| Cotton (fabric) | 0.77 |
| Glass | 0.92 |
| Glass (polished) | 0.94 |
| Gold (polished) | 0.018 - 0.035 |
| Grass | 0.76 - 0.74 |
| Human skin | 0.98 |
| Ice | 0.97 |
| Marble, white< | 0.95 |
| Sand | 0.76 |
| Silver (polished) | 0.02 - 0.03 |
| Snow | 0.99 |
| Stainless steel (polished) | 0.075 |
| Stainless steel 301 | 0.54 - 0.63 |
| Teflon (coating) | 0.38 |
| Tile | 0.97 |
| Water | 0.95 - 0.963 |
| Wood (untreated) | 0.90 - 0.95 |
Emissivity of various materials, Source: Wikipedia
Thus a thin aluminized sheet prevents heat loss of an injured person.


