Homo sapiens sapiens is the only specie on Earth that is knowingly exploiting natural resources on a global scale.
While for a long time the forces of nature were considered as overwhelming, various hypothesis are now claiming that human activities have an irreversible impact on the planet, in particular by precipitating a change of the climate. This is the so-called anthropogenic hypothesis.
Landscaping by agriculture, industrialisation and urbanisation
According to FAO, from the total Earth surface of 510’072’000 km2 (1 km2 = 100 Ha = 247.1 acre):
- one fourth is land area,
- one tenth is agricultural area, and
- 3% is arable land and permanent crops.
Deforestation, in particular in tropical zones, have led to substitution of wild species by agricultural crops or other managed forestry. Also, the changed vegetation that grows on such surface reflects and absorbs the solar irradiation in a different way. Depending on available nutrients the intensity of the photosynthesis will also change.
According to UNEP the built-up area made of residential and industrial building as well as transportation routes, represents approx. 2% of the land area on Earth, or 0.6% of the surface of the globe.
While it makes no doubt that local climatic conditions have been altered by these human interventions (so called micro-climates) no global data exists about consequences on global climate.
Emission of dust and other substances
Nature and humans are emitting dust and particulates (e.g. sulfates) into the atmosphere. Global quantitative measurements beyond the transmittance diagram shown on Figure 13 are not available, in particular there are no way to distinguish human from natural sources, even in local monitoring of air pollution.
The only synthetic gases for which a global impact could be assessed are the chlorofluorocarbons (CFC) used in refrigeration machines that affect the stratospheric ozone layer over the poles, and therefore reduce the sunlight absorption in the ultraviolet range.
Volatile Organic Chemicals (VOC) are emitted into the atmosphere by plants, animals and by human industry in the form of evaporated solvents and other chemicals used in various applications such as surface treatment, printing, dry cleaning, etc. Once in air, they slowly decompose by oxidation under the influence of sunlight.
Combustion of fossil fuels
Fossil fuels are either coal, a solid made of elemental carbon, or hydrocarbons in the liquid (oil) or gaseous state. Oxygen is required to burn fossil fuels. The combustion reaction produces heat, energy that is used to warm buildings, to run chemical processes, and to be transformed into mechanical or electrical energy.
Hydrocarbon + Oxygen → Carbon dioxide + Water + combustion energy
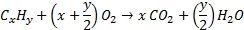
Partially burned hydrocarbons as well as unburned gases are also emitted into the atmosphere. Together with VOCs they will degrade by reacting with atmospheric oxygen.
In parallel with the burning process, nitrogen oxides are formed, in particular at high temperature, in combustion engines and incinerators. Under the influence of ultraviolet light they will further form ozone, a respiratory irritant.
Combustion is not only a human activity. Nature constantly produces combustible substances, in particular carbohydrates, wood and VOCs, that will eventually also be burned or fermented to produce CO2.
Photosynthesis is the reverse process to combustion that uses light as energy source and chlorophyll as catalyst; it consumes carbon dioxide and fixes it in form of biomass:
Carbon dioxide + Water → Carbohydrate + Oxygen
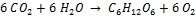
Energy involved in producing biomass is relatively modest as compared to solar irradiation. When one hectare of forest produces 30 metric tons of wood per year this means that the photosynthesis is converting 1.7 W m-2, or 0.5% of the total incoming sunlight. Photosynthesis serves the plant for its growth and its reproduction; to this effect, energy conversion does not need to be maximized.
Biomass growth is not constrained by the solar energy input but by the plant genetics, competition for space (in the soil and above ground), the rate of absorption and the quantity of available nutrients (with the Liebig limiting factor of the least abundant of N, P or K), irrigation, and, last but not least, the concentration of atmospheric CO2 to engage photosynthesis.
Atmospheric CO2
Carbon dioxide is produced by the combustion of hydrocarbons and biomass. Aerobic fermentation, a slower way to burn combustibles, also produces CO2. Also, in the production of cement, CO2 will be separated from calcium carbonate to form lime. Therefore, cement kilns emit CO2 in two ways: by burning fuel to heat the kiln, and by the cement making process itself. When concrete is made, a long process of carbonation is then induced that will slowly capture back part of the emitted CO2.
Since 1960, the Mauna Loa observatory in Hawaii is measuring the atmospheric CO2 concentration by the precise method of IR absorption. The waves in the following figure correspond to seasonal variations, growth and decay of biomass in the Northern hemisphere where more landmass and vegetation is present than in the Southern hemisphere.
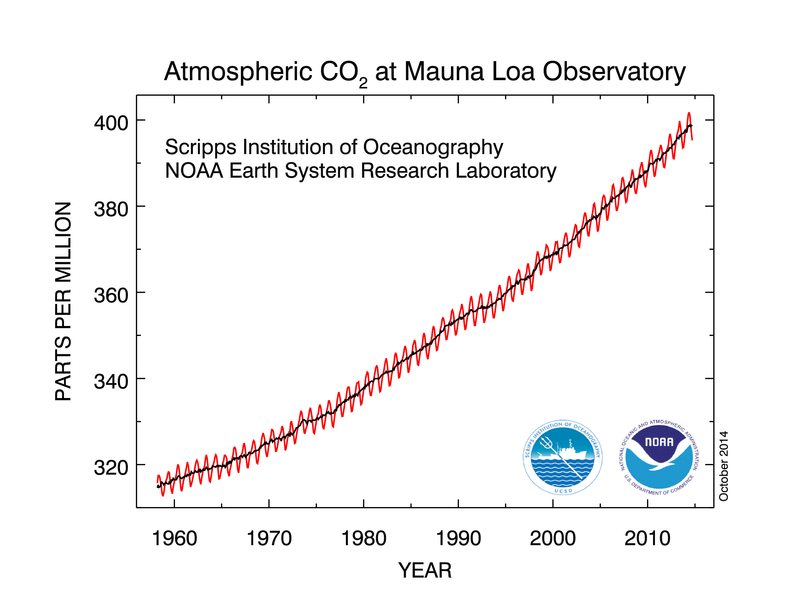
CO2 atmospheric concentration in Mauna Loa, Hawaii. Source: NOAA
This well-known chart represents only the concentration of carbon dioxide at this Hawaiian site. Additional data is required to get global view at the Earth surface, but the pattern remains similar.
On average, the CO2 concentration currently increases by 2.5 ppm every year, or with a compounded annual growth rate (CAGR) of 0.67%.
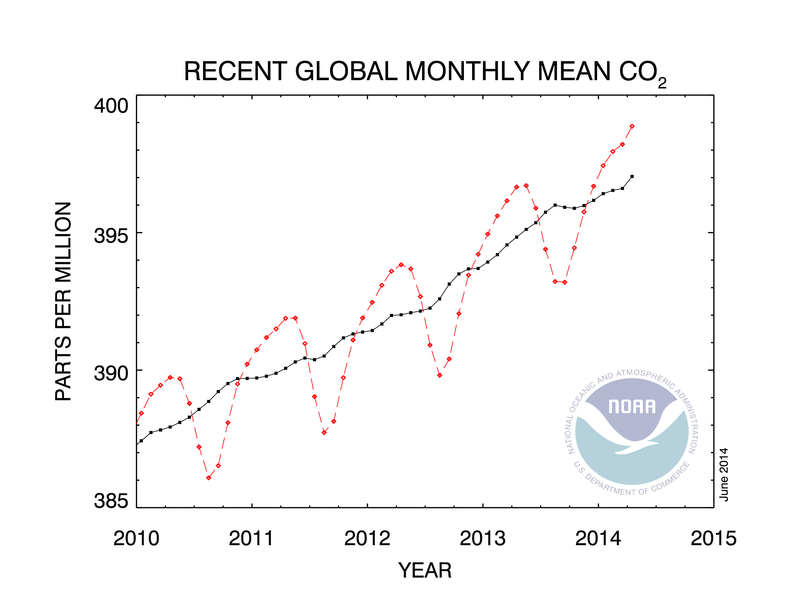
Global mean CO2 concentration at the earth surface (source NOAA)
Prior to 1960, measurements were made by chemical analysis at various locations. Paleo-climatic data are based on the analysis of gas bubbles trapped in ice sample taken at various glacier depths.
In pre-industrial times the CO2 concentration was quite stable at around 280 ppm:
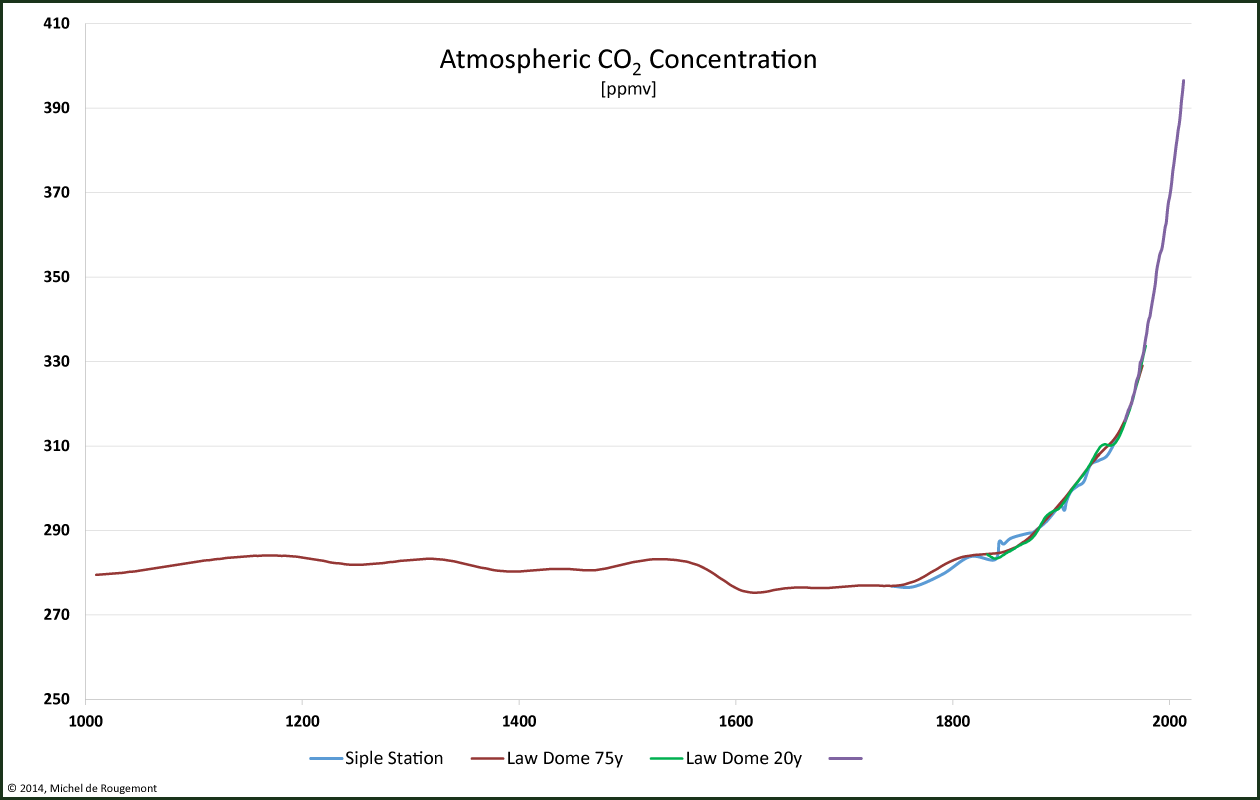
Historic atmospheric CO2 concentration.
Sources: D.M. Etheridge et al., CDIAC
(please note the ordinate scale: the origin is not at 0)
Before the beginning of the industrial era (BIE) no large quantities of fossil fuels were extracted from the Earth. Records of consumption have been reconstructed for the period covering 1750 to present time.
In 2012 the yearly emissions of total carbon were:
|
Total |
Carbon emissions from gas fuel consumption |
Carbon emissions from liquid fuel consumption |
Carbon emissions from solid fuel consumption |
Carbon emissions from cement production |
Carbon emissions from gas flaring |
|---|---|---|---|---|---|
|
9’776 |
1’776 |
3’185 |
4’137 |
509 |
59 |
Total carbon emission in 2012, in million metric tons of C, or teragram Tg C.
To get CO2 quantities multiply by 3.664 as carbon’s atomic weight is 12.01 and CO2 molecular weight is 44.01.
Source: CDIAC
Cumulated since the BIE it is estimated that a total of 384 Pg (petagram or billion metric tons) have been emitted. These are 3.20·1016 moles of C or CO2.
The compounded growth rate of the yearly emissions over the period 2002-2012 has been 3.3% with only the year 2009 in decline, probably due to the economic and financial crisis.
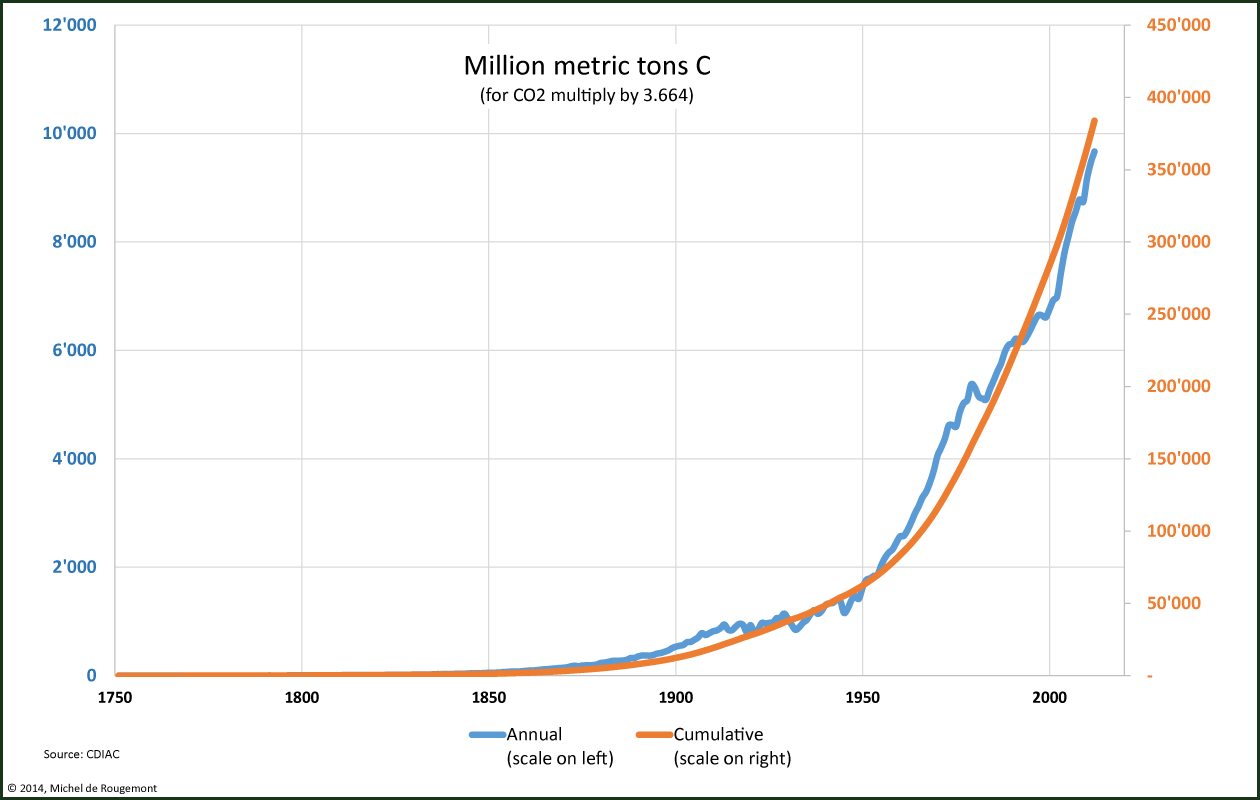
Carbon emissions from fossil combustion and cement kilns
Source: cdiac.ornl.gov
There is quite a similarity with the increase of the CO2 atmospheric concentration:
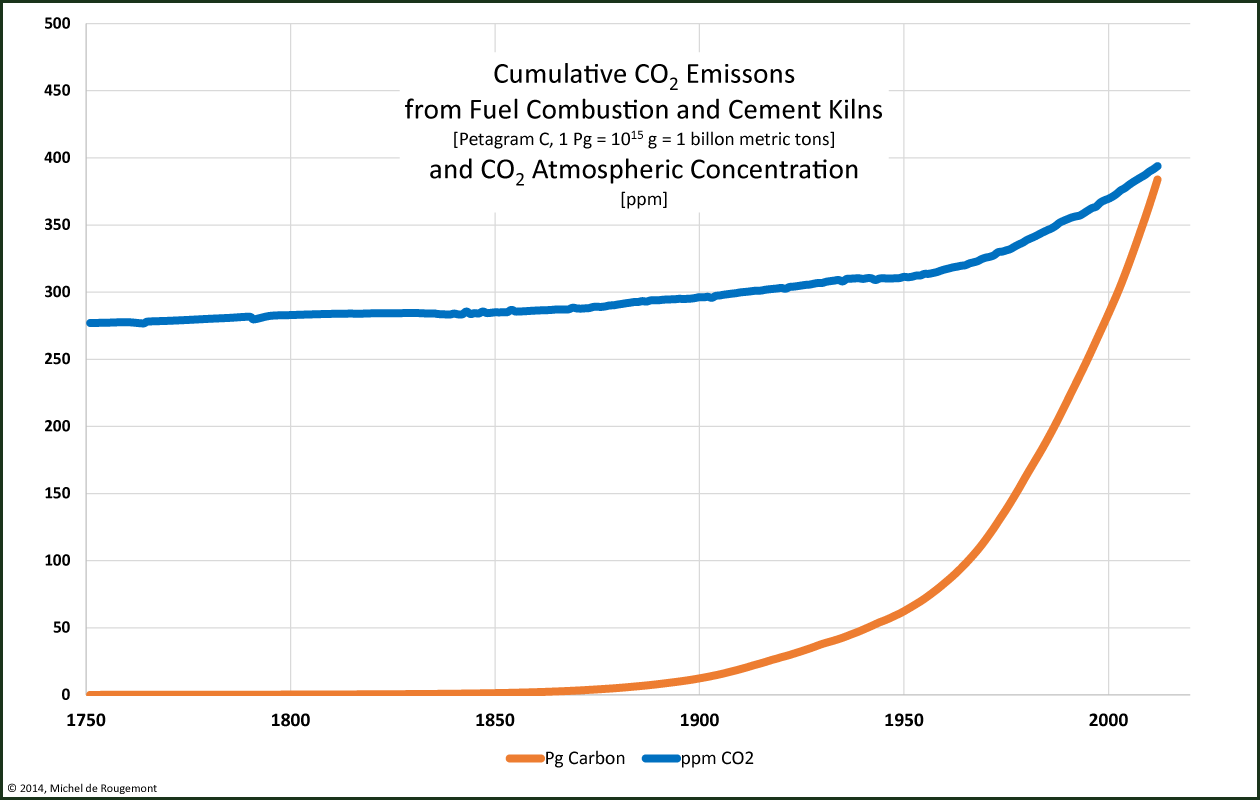
Cumulated carbon emissions since 1750 (in petagram) and
atmospheric CO2 concentration in ppm (by volume)
Methane
Methane is the product of anaerobic fermentation (in absence of air) taking place in nature and influenced by agricultural and industrial activities. Its current concentration is 1.8 ppm, 2.5 times higher than its historic concentration. This low absolute concentration can be explained by the high reactivity between methane and air; its residence time in atmosphere is therefore much shorter than the chemically more stable CO2. Atmospheric methane is also provided by natural sources such as wetlands, termites, and geological deposits. It is estimated that 60-70% of the total emissions are caused by agriculture (ruminants and rice farming), coal mining, oil and gas extraction, and biomass partial combustion.
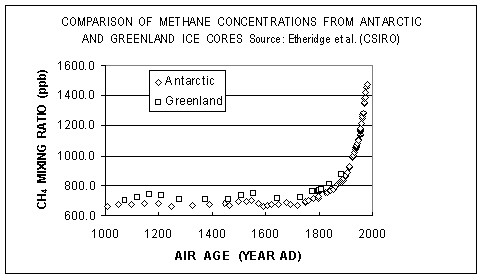
Methane concentration from ice core samples
Source: CDIAC
Recent data show some flattening of the concentration:
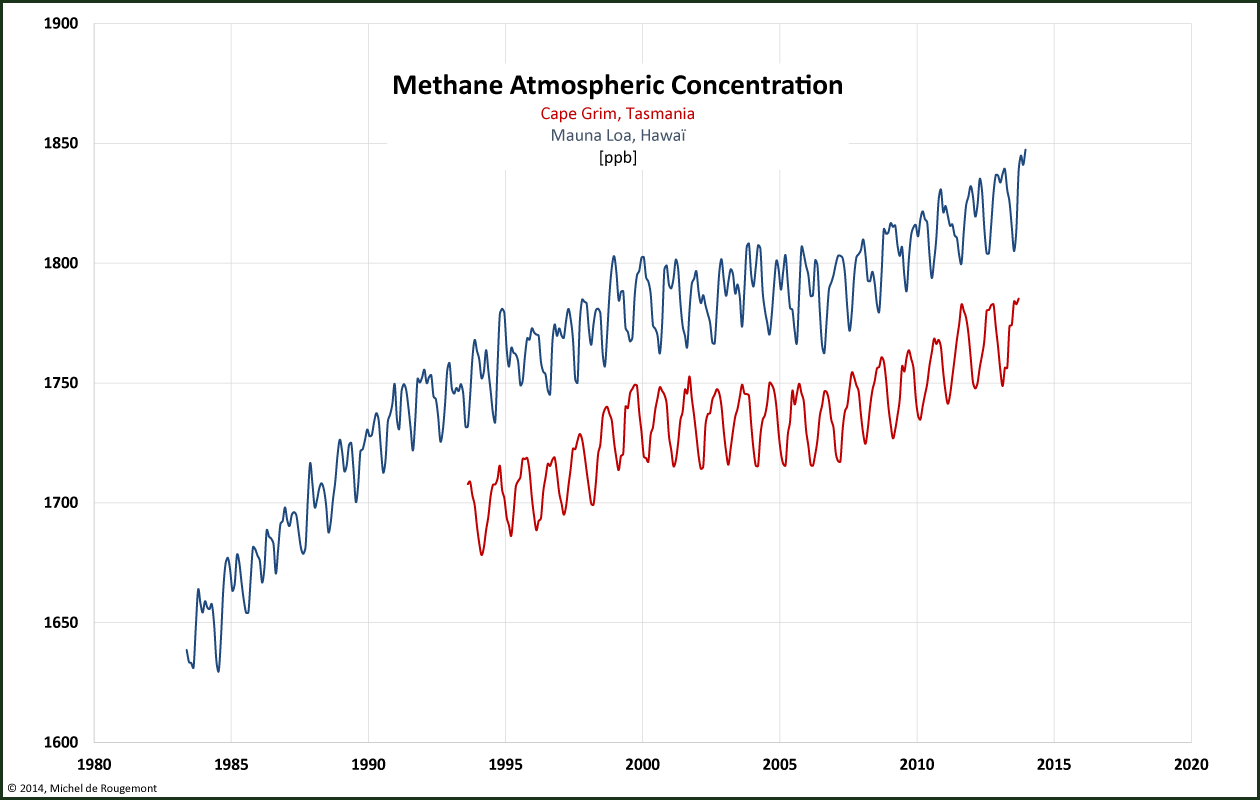
Atmospheric Methane Dry Air Mole Fractions in ppb.
Source: http://agage.eas.gatech.edu/data_archive/agage/gc-md/monthly/
and NOAA, http://www.esrl.noaa.gov/gmd/dv/data/index.php?site=mlo
Nitrous oxide N2O
Nitrous oxide is formed during combustion of any fuel by the reaction of nitrogen (80% in air) with oxygen at high temperature.. It is also a reaction product of volatile organic compounds (naturally occurring or artificially emitted) with oxygen under the influence of sunlight. It is also associated with the production and use of fertilizers. Here too, its instability explains the low concentration level. The historic pre-industrial level is estimated from ice core samples at 270 ppb.
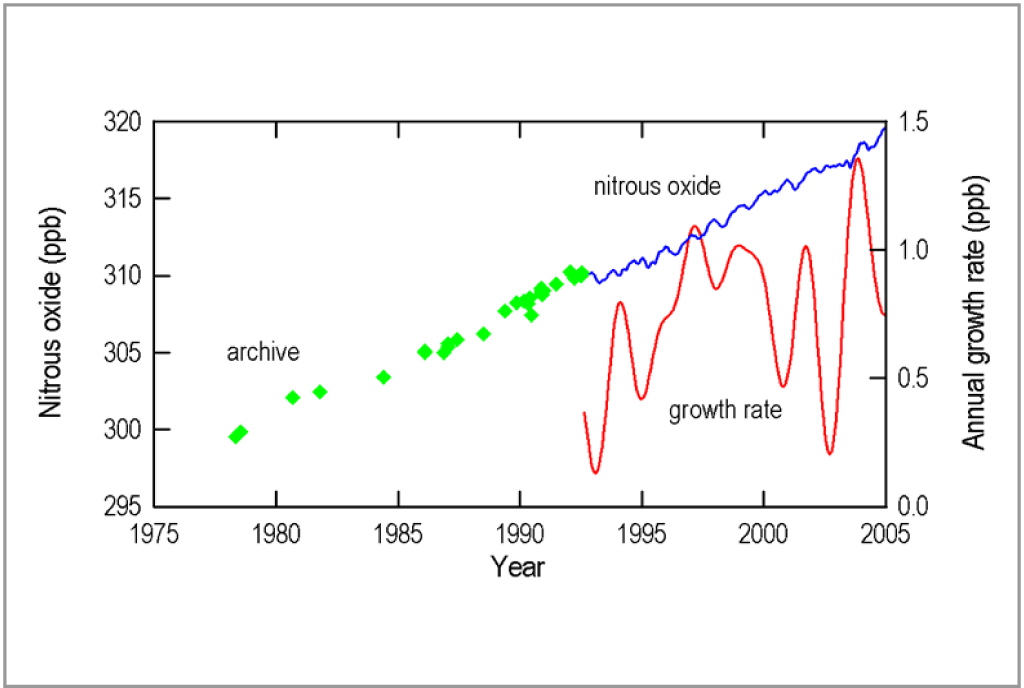
Recent evolution of N2O concentration
Source: CSIRO Atmospheric Research and Cape Grim Baseline Air Pollution Station
Australian Bureau of Meteorology


