Radiative heat transfer
According to Planck’s and Stefan-Boltzman’s laws, bodies emit electromagnetic radiation in dependence on their temperature.
Any surface which temperature is above 0 K emits energy. By doing so it loses some of the heat contained in the material of which it is made. The speed at which such cooling takes place is limited by the conductivity of the solid material, or of by the flow condition of water at the surface of the sea. When the surface temperature reaches the thermal equilibrium, as much energy is radiated as it is received from the surrounding environment.
According the 1st law of thermodynamics, a material cannot give away more heat that it contains.
Sensible heat, heat content or latent heat: this quantity depends on the mass and on the specific heat capacity of the material. Heat involved in a change of its temperature is calculated as:
![]() (eq. 2)
(eq. 2)
Where
- Q is the total heat implied in the change [J]
- m is the mass of the material [kg]
- CP is the specific heat capacity at constant pressure [kJ kg-1 K-1]
- Ice at 0 °C 2.05
- Water at 15 °C 4.186
- Air at -50 to +40 °C 1.005
- T1 and T0 are the temperature after and before the change [K]
Melting - Crystallisation, Evaporation – Condensation: heat is being transferred by the change of the physical state of the material.
For climate considerations, water is the vehicle of such processes:
- Latent heat of fusion of water at 0 °C: 334 [ kJ kg-1 ]
- Latent heat of vaporisation of water at 100 °C and normal pressure: 2257 [ kJ kg-1 ]
- Latent heat of vaporisation of water at 14 °C and normal pressure: 2467 [ kJ kg-1 ]
Planck’s Law
Planck’s law defines the radiance, the quantity of radiation that passes through or is emitted from a surface when in thermal equilibrium at a definite temperature.
At a given wavelength the radiance will be calculated as:
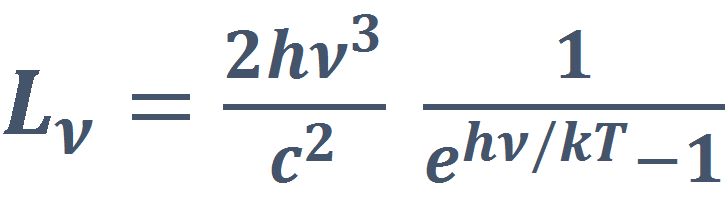 [W m-2 sr-1 Hz-1 = W m-2 sr-1 s] (eq. 3)
[W m-2 sr-1 Hz-1 = W m-2 sr-1 s] (eq. 3)
where:
- h is Planck’s constant [6.6260693×10−34 W s2]
- c is the speed of light [2.99792458×108 m s−1]
- k is Boltzmann’s constant [1.380658×10−23 J K−1]
- ν = λ/c [s-1], where λ is the wave length [m].
Solving this over a range of wavelengths for a temperature of 5’776 K gives the following spectra:
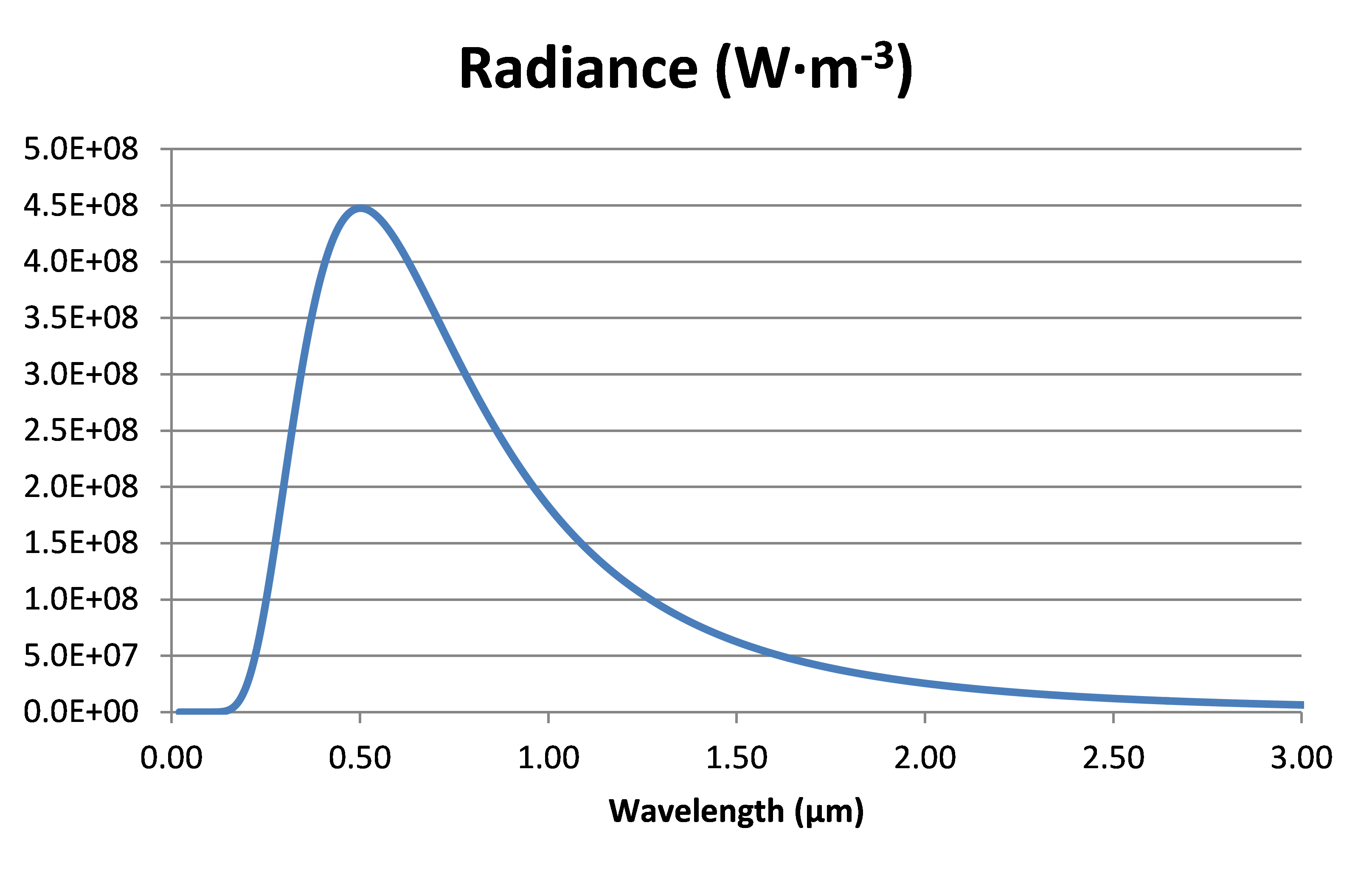
Radiance of a black body at 5776 K, the so-called effective temperature of the sun.
The wavelength at which there is a maximum is calculated by the Wien’s law:
In the case of the sun at 5776 K, λmax = 0.502 µm; this is within the visible range of light (0.38-0.75 µm).
At the surface temperature of the earth of 288 K (15 °C) the radiance spectra will be:
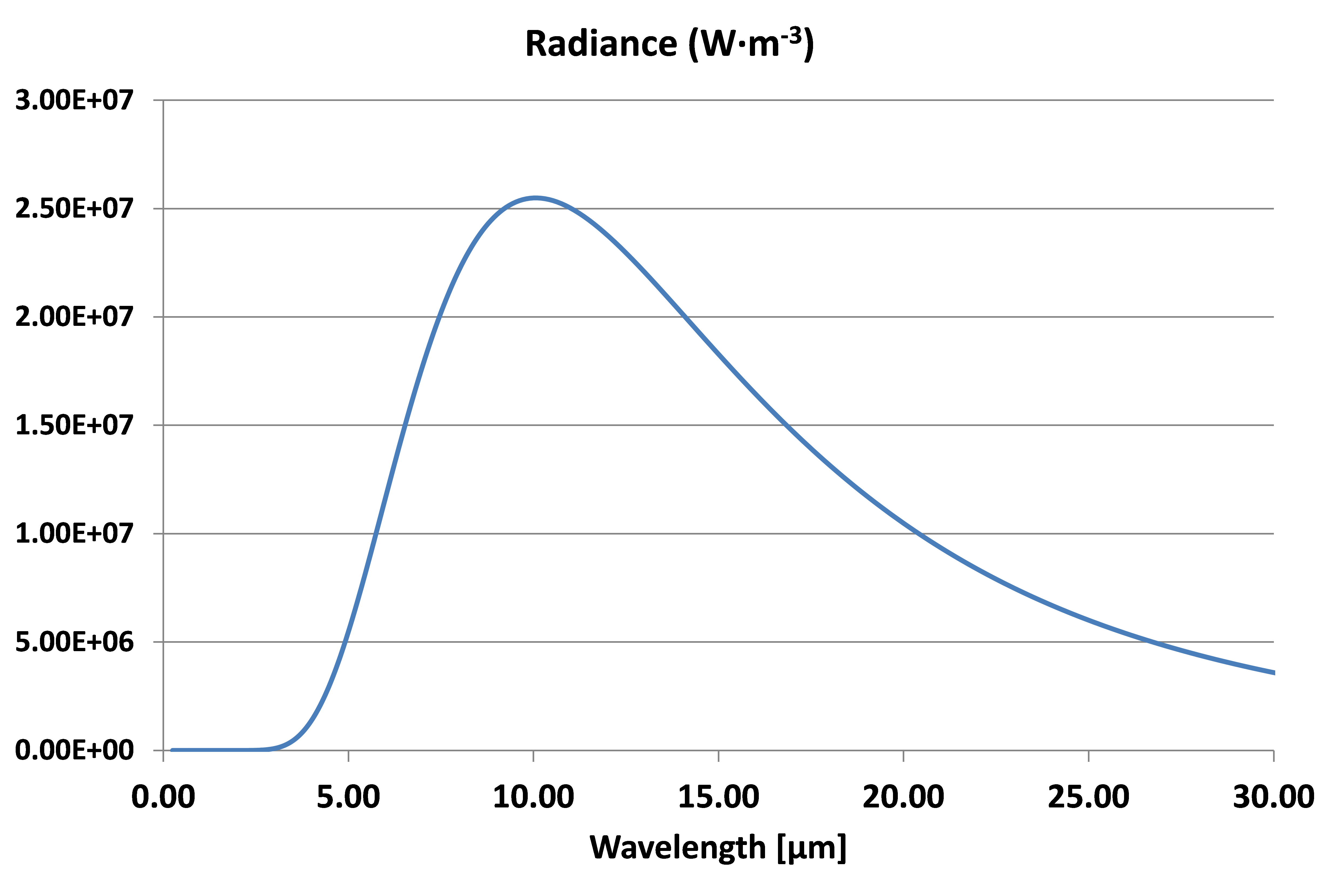
Radiance spectra at T =288 K
In this case the peak is at λmax = 10.06 µm, in the non-visible infrared range.
Stefan Boltzman Law
This laws integrates Plank’s law and defines the electromagnetic power that is emitted by the surface of a lump of matter in relation with its temperature.
![]() (eq. 4)
(eq. 4)
Where
- E is the flux of energy [W m-2]
- ε is the emissivity of the black body (1 if perfect) [-]
- σ is the Stefan Boltzman constant 5.670·10-8 [W· m-2· K-4]
- T is the temperature [K]
Thus at 5’776 K the sun emits 63.11 MW m-2.
And the earth, at its hypothetical 288 K and if a perfect black body, emits 390 W m-2.
Far away from us, at the temperature of the cosmic microwave background radiation of 2.7 K, the radiation is a mere 3 µW·m-2.
The emissivity factor of 1 applies only for a hypothetical perfect black body. Reality of matter is other:
|
Material |
Emissivity |
|
Aluminium (anodized) |
0.77 |
|
Aluminium (polished) |
0.039 - 0.057 |
|
Asphalt |
0.93 |
|
Carbon (graphite) |
0.98 |
|
Clay (baked) |
0.91 |
|
Concrete |
0.92 |
|
Cotton (fabric) |
0.77 |
|
Glass |
0.92 |
|
Glass (polished) |
0.94 |
|
Gold (polished) |
0.018 - 0.035 |
|
Grass |
0.76 - 0.74 |
|
Human skin |
0.98 |
|
Ice |
0.97 |
|
Marble, white |
0.95 |
|
Sand |
0.76 |
|
Silver (polished) |
0.02 - 0.03 |
|
Snow |
0.99 |
|
Stainless steel (polished) |
0.075 |
|
Stainless steel 301 |
0.54 - 0.63 |
|
Teflon (coating) |
0.38 |
|
Tile |
0.97 |
|
Water |
0.95 - 0.963 |
|
Wood (untreated) |
0.90 - 0.95 |
Table 3 Emissvity of various materials
Source: Wikipedia
This explains why a thin aluminized sheet prevents heat loss of an injured person.


